Exploring the World of Stem Cells: A Guide to Our Product Range
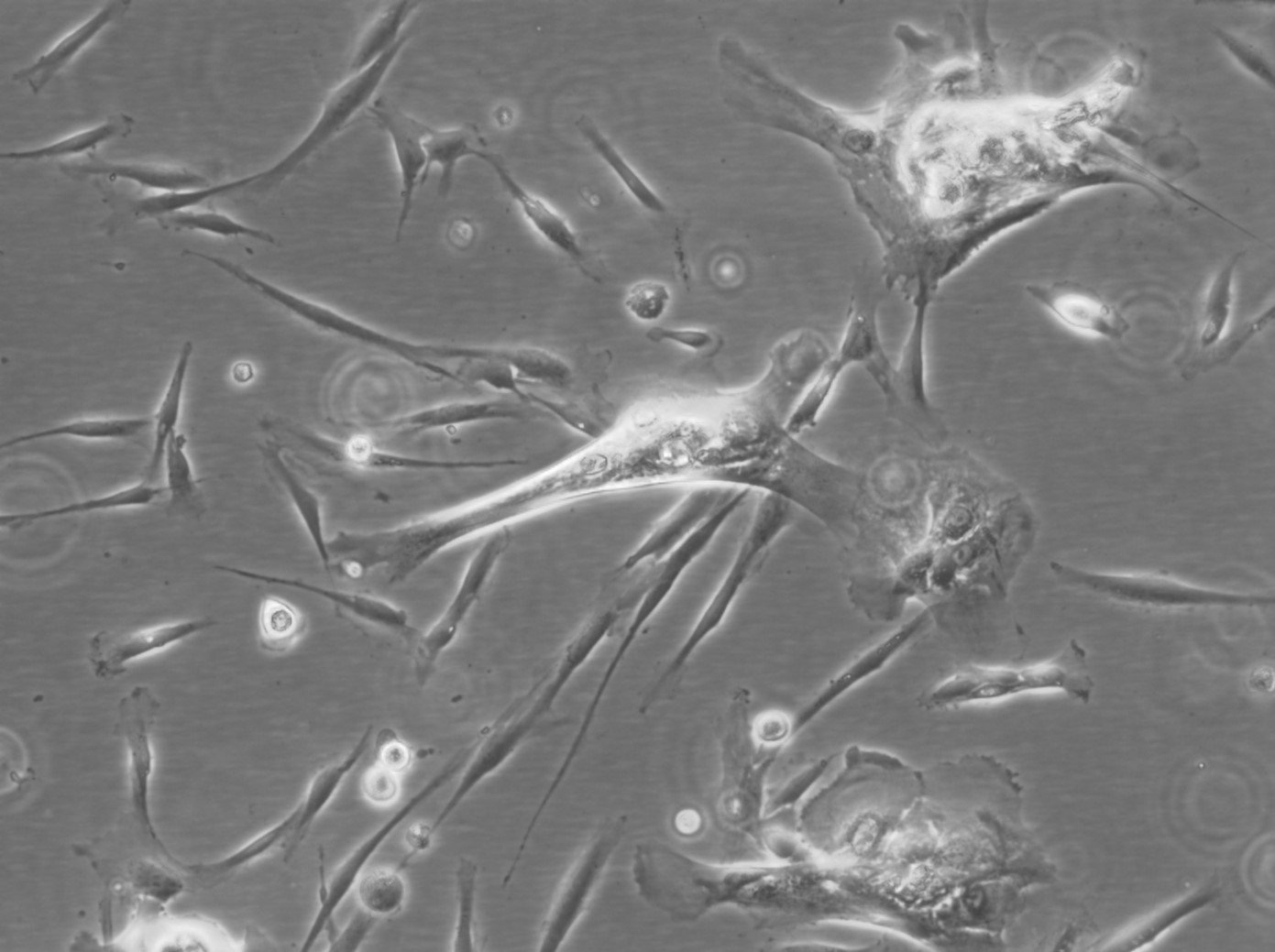
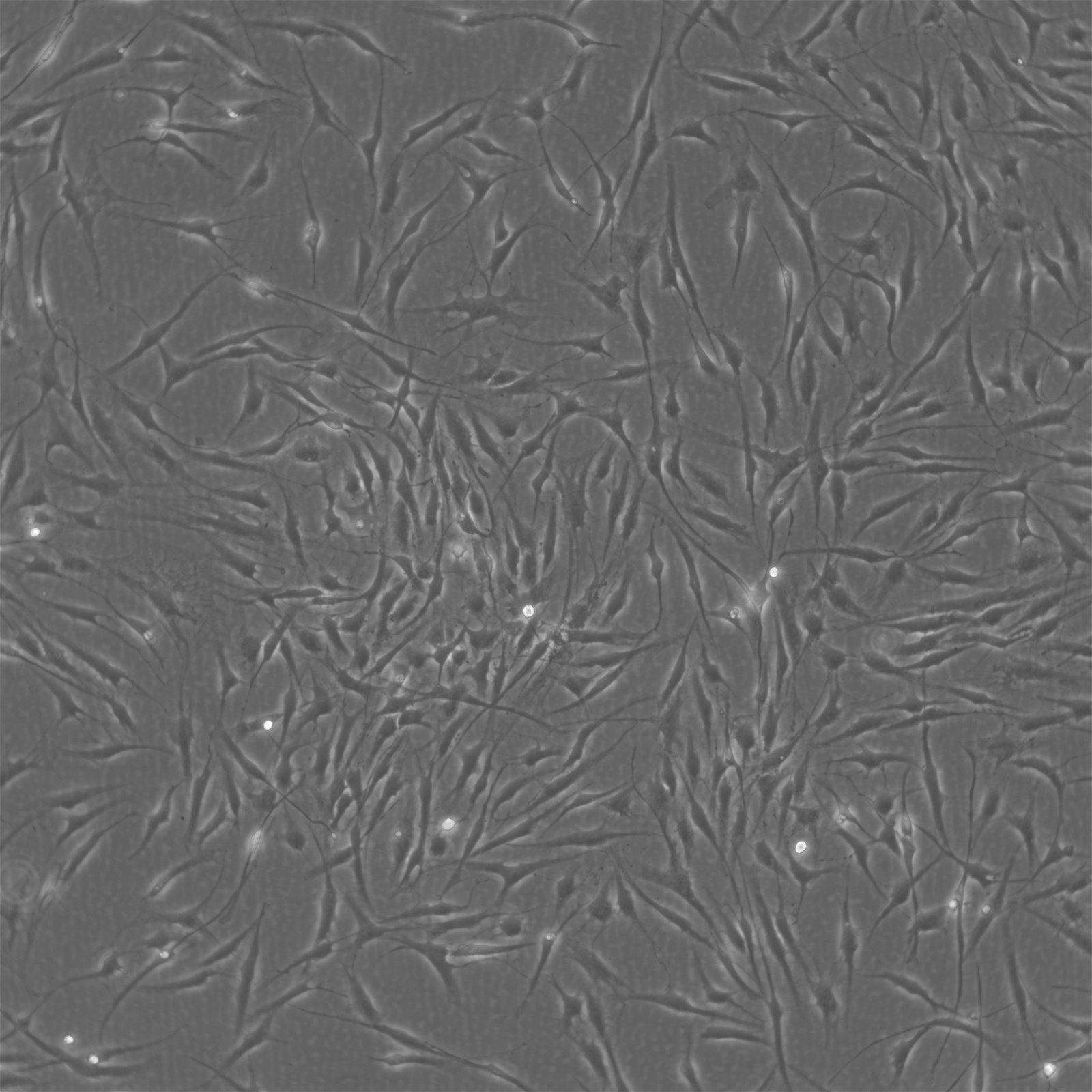
Stem cells, the building blocks of life, have revolutionized the world of regenerative medicine and biological research. Known for their self-renewing and multipotent characteristics, are a cornerstone in cell therapy and regenerative medicine. These cells showcase remarkable versatility, differentiating into adipocytes, osteoblasts, and chondrocytes. This differentiation is facilitated through specialized media in vitro, highlighting MSCs' potential in diverse medical applications. At Cytion, we proudly offer a diverse array of human mesenchymal stem cells (hMSCs) and human dental follicle stem cells (hDFSCs), catering to various research and therapeutic needs.
Stem Cell Portfolio
At Cytion, our product portfolio includes stem cells derived from various human tissues:
- Human Mesenchymal Stem Cells - Bone Marrow (HMSC-BM): Sourced from bone marrow, these cells are fundamental in hematological and orthopedic research.
- Human Mesenchymal Stem Cells - Umbilical Cord - Artery: Derived from the umbilical cord artery, these cells are pivotal for studies in vascular biology and regenerative medicine.
- Human Mesenchymal Stem Cells - Endometrium: Extracted from the endometrial tissue, these cells are essential for reproductive health research.
- Human Mesenchymal Stem Cells - Chorion Villi: Sourced from the chorion villi, they are vital for prenatal development studies.
- Human Mesenchymal Stem Cells - Amnion: Derived from the amnion, these cells hold potential for wound healing and tissue engineering applications.
- Human Mesenchymal Stem Cells - Adipose Tissue: Extracted from adipose tissue, they are crucial in obesity and metabolic studies.
- Human Mesenchymal Stem Cells - Wharton's Jelly (HMSC-WJ): Derived from the Wharton's Jelly of the umbilical cord, these cells are important for neonatal health research.
- Human Dental Follicle Stem Cells (hDFSC): Sourced from dental tissue, these cells are significant in dental and craniofacial research.
Quality Control
>Our MSCs are derived from donors who have provided informed consent. They undergo stringent quality control checks to ensure their viability and appropriateness for research and clinical applications. Early passage MSCs are cryopreserved using a specific cryomedium, ensuring a minimum viability of 92% to 95% post-thawing.
Marker Panels
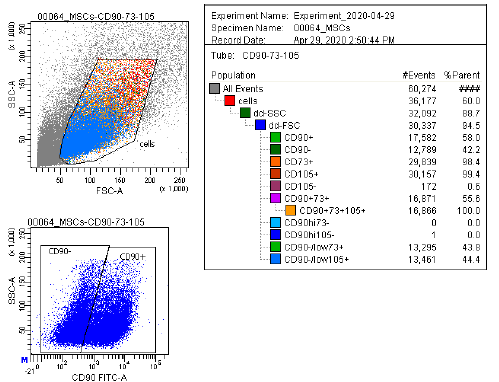
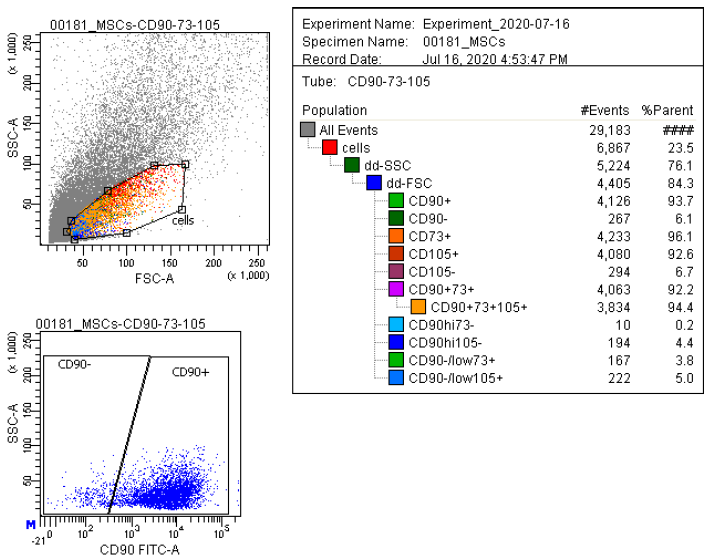
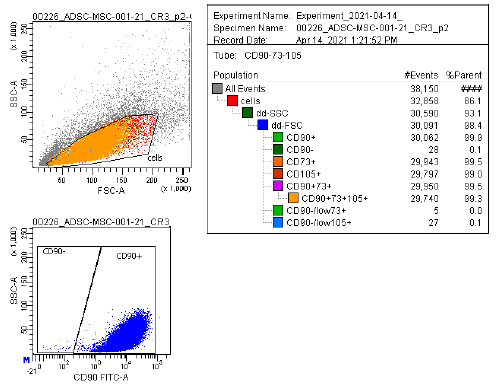
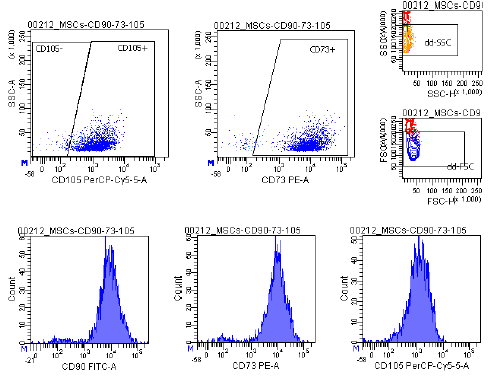
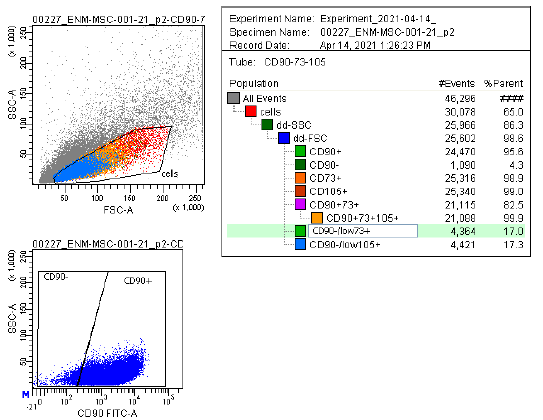
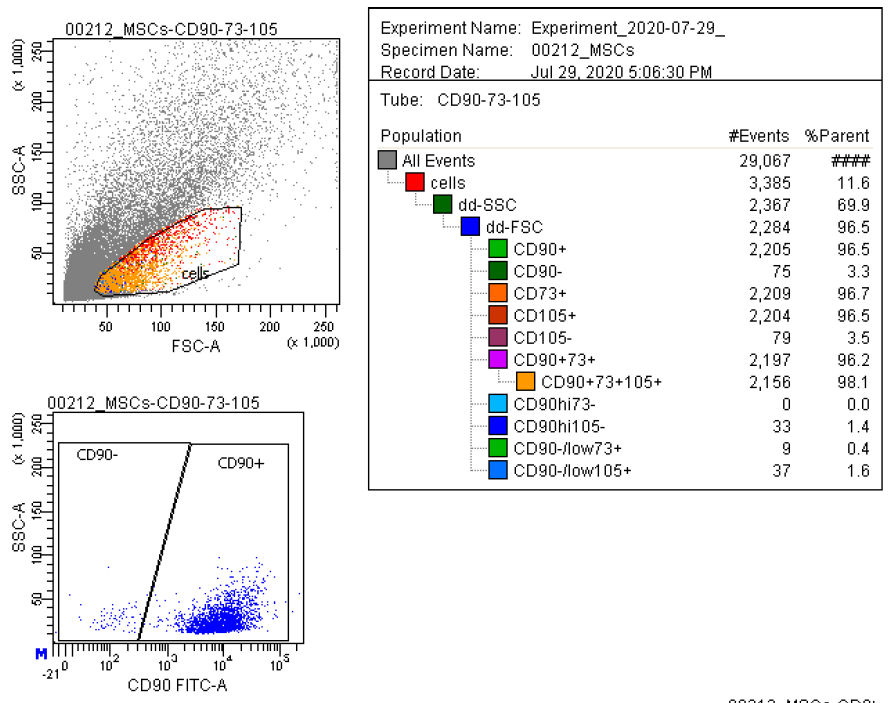
A comprehensive panel of markers, including CD73/CD90/CD105 (positive) and CD14/CD34/CD45/HLA-DR (negative), is utilized in flow cytometry analysis for identifying cultivated MSCs prior to cryopreservation. This process adheres to the recommendations of the ISCT MSC committee.
Safety and Purity
Our stringent safety measures include testing each donor for HBV, Treponema pallidum, and HIV-1/2. The cells are additionally screened for a range of viruses and pathogens, including HBV, HCV, HSV1, HSV2, CMV, EBV, HHV6, Toxoplasma gondii, Treponema pallidum, Chlamydia trachomatis, Ureaplasma urealyticum, and Ureaplasma parvum, ensuring the highest standards of safety and purity.