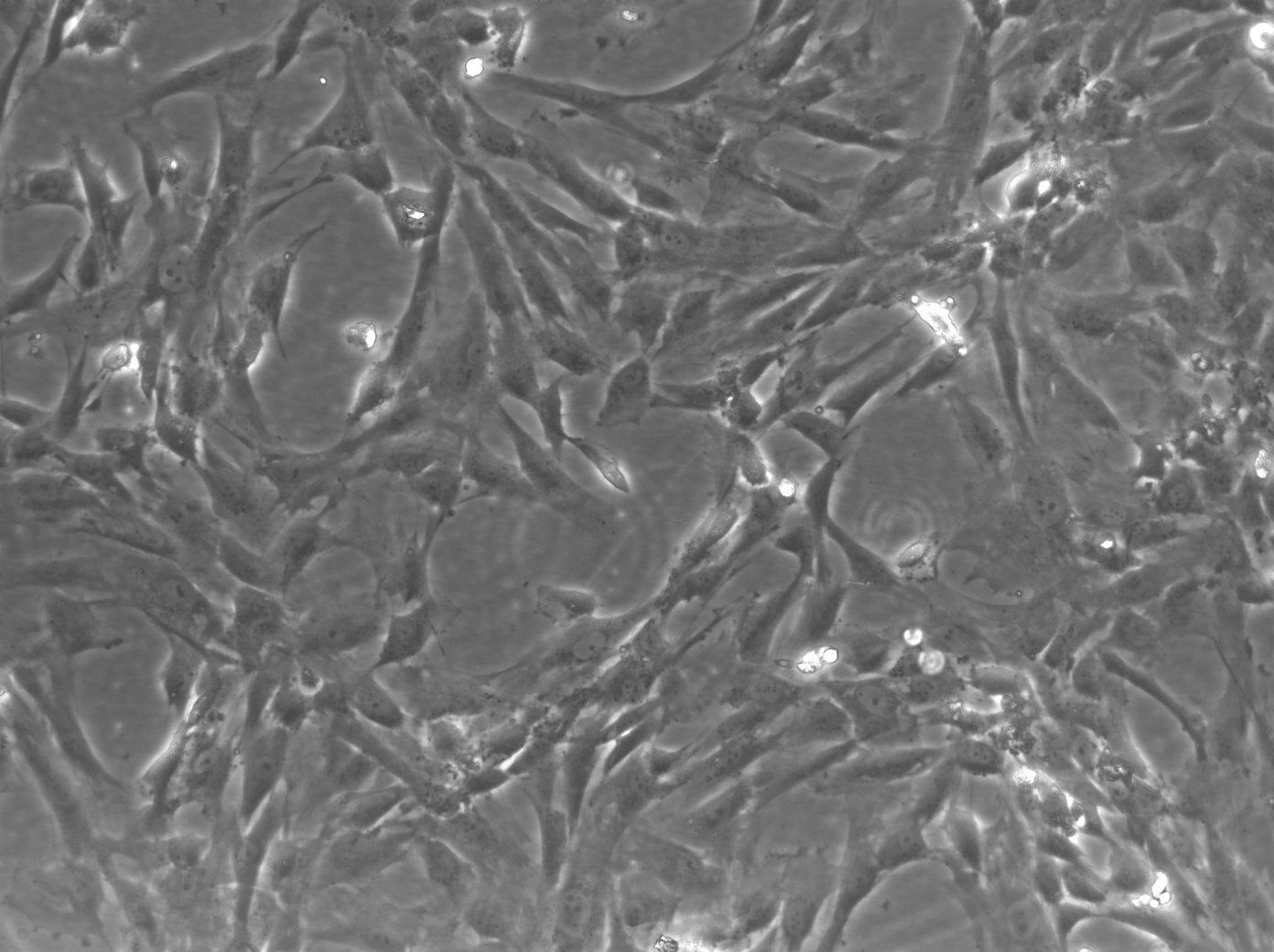
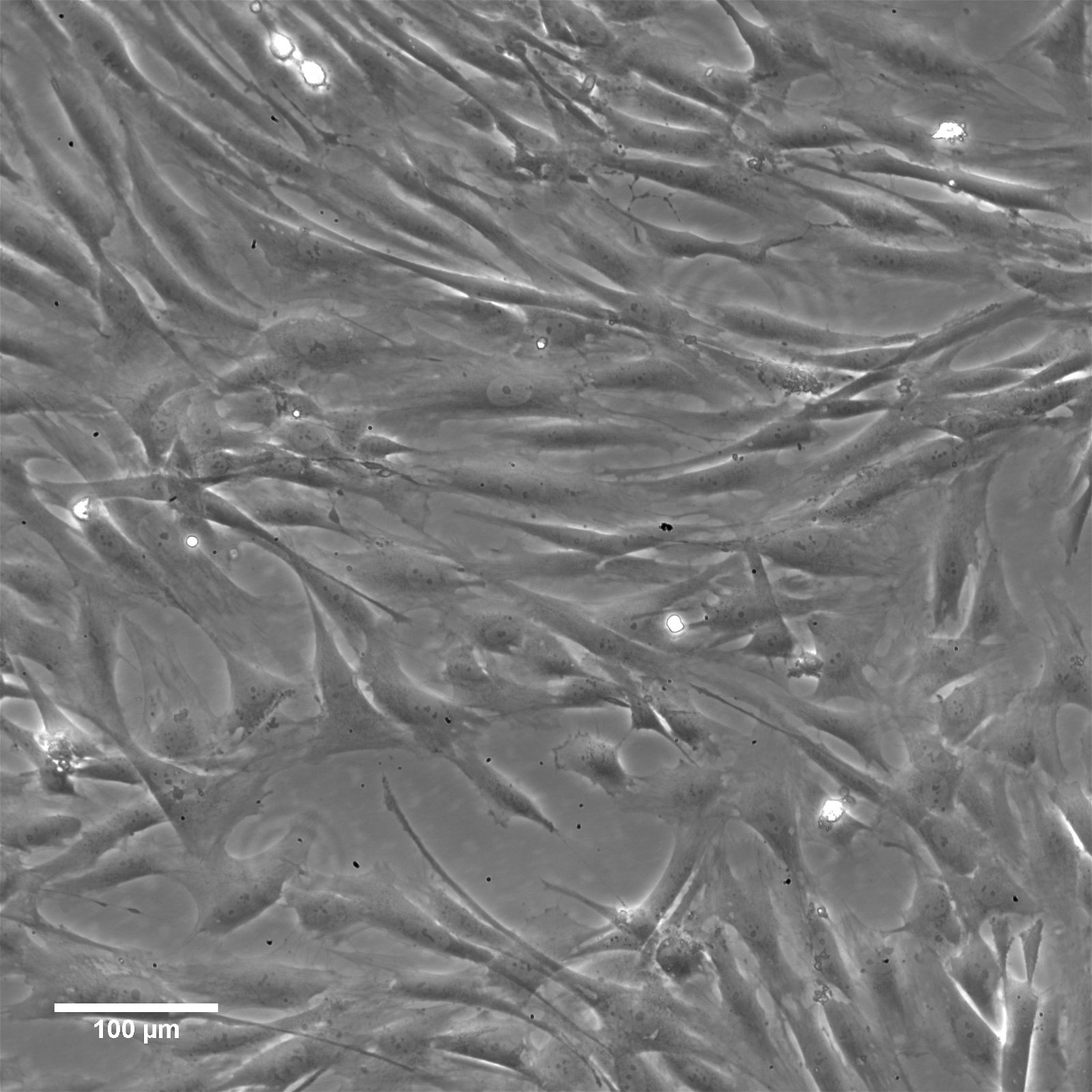
imWilms1 Cells

€600.00*
Prices excl. VAT plus shipping costs This cell line is licensed to CLS by a university or institute. The sale of this item requires the conclusion of a Material Transfer Agreement (MTA). Please get in touch with us for further information.
General information
| Description | The imWilms1 cell line was immortalized from Wilms1 cells, a primary tumor cell line which was established in 2010 by the group of Dr. Brigitte Royer-Pokora from tissue of a 10-month-old female patient with large bilateral kidney tumors. Immortalization using triple mutant (U19dl89-97tsA58) SV40 large T antigen (LT) resulted in the chromosomally stable cell line imWilms1. |
|---|---|
| Organism | Human |
| Tissue | Kidney |
| Disease | Wilms Tumor |
| Synonyms | IM-WT-1 |
Characteristics
| Age | 10 months |
|---|---|
| Gender | Female |
| Ethnicity | Caucasian |
| Morphology | Spindle-shaped |
| Cell type | Wilms cells |
| Growth properties | Adherent |
Identifiers / Biosafety / Citation
| Citation | imWilms1 (Cytion catalog number 300412) |
|---|---|
| Biosafety level | 1 |
| Depositor | B. Royer-Pokora |
Expression / Mutation
| Mutational profile | WT1 mutation status: homozygous c. 149 C>A, p.S50x, LOH: 11p11-11pter, CTNNB1 mutation status: heterozygous TCT>TTT, p.S45F |
|---|
Handling
| Culture Medium | MSCGM kit (from Lonza) |
|---|---|
| Passaging solution | Accutase |
| Subculturing | Remove the old medium from the adherent cells and wash them with PBS that lacks calcium and magnesium. For T25 flasks, use 3-5 ml of PBS, and for T75 flasks, use 5-10 ml. Then, cover the cells completely with Accutase, using 1-2 ml for T25 flasks and 2.5 ml for T75 flasks. Let the cells incubate at room temperature for 8-10 minutes to detach them. After incubation, gently mix the cells with 10 ml of medium to resuspend them, then centrifuge at 300xg for 3 minutes. Discard the supernatant, resuspend the cells in fresh medium, and transfer them into new flasks that already contain fresh medium. |
| Fluid renewal | 1 to 2 times per week |
| Freeze medium | CM-1 (Cytion catalog number 800100) or CM-ACF (Cytion catalog number 806100) |
| Handling of cryopreserved cultures |
|
Quality control / Genetic profile / HLA
| Sterility | Mycoplasma contamination is excluded using both PCR-based assays and luminescence-based mycoplasma detection methods. To ensure there is no bacterial, fungal, or yeast contamination, cell cultures are subjected to daily visual inspections. |
|---|---|
| STR profile |
Amelogenin: x,x
CSF1PO: 10,12
D13S317: 11,13
D16S539: 11,14
D5S818: 12,13,14
D7S820: 9,14
TH01: 9.3
TPOX: 8,9
vWA: 14,19
D3S1358: 14,17,18
D21S11: 30,31
D18S51: 15,18
Penta E: 5,14
Penta D: 13
D8S1179: 12,14
FGA: 22,25
|
| HLA alleles |
A*: 03:01:01, 24:02:01
B*: 35:03:01, 38:01:01
C*: 12:03:01
DRB1*: 07:01:01, 14:54:01
DQA1*: 01:04:01, 02:01:01
DQB1*: 02:02:01, 05:03:01
DPB1*: 02:01:02G, 04:02:01G
E: 01:03:01, 01:03:02
|
Required products
In biological research, the cryopreservation of mammalian cells is an invaluable tool. Successful preservation of cells is a top priority given that losing a cell line to contamination or improper storage conditions leads to lost time and money, ultimately delaying research results. Once the cells have been transferred from a cell growth medium to a freezing medium, the cells are typically frozen at a regulated rate and stored in liquid nitrogen vapor or at below -130°C in a mechanical deep freezer. The freeze medium CM-1 enables cryopreservation of cells at below -130°C (or in liquid nitrogen), essentially eliminating the need for an additional, costly ultralow freezer and eliminating time-consuming and demanding controlled rate freezing processes. Simply collect the cells, aspirate the growth medium, resuspend in CM-1, transfer to a cryovial, and store the vial at below -130 °C.
Long shelf-life
CM-1 is a serum-containing, ready-to-use cryopreservation medium that can be stored in the refrigerator for up to one year.
Trusted by hundreds of researchers
Our advanced cell freezing medium CM-1 is a market-leading product in Germany and Europe and is distinguished by numerous publications involving hundreds of different cell lines worldwide. We tested it with more than 1000 cell lines from our proprietary cell bank.
Optimized ingredients
CM-1 does contain serum products. Serum-containing cryopreservation mediums optimally protect the cells whilst being frozen and have the advantage of high recovery rates. As CM-1 has been tested with a multitude of cell lines, you can rest assured that your cells always recover well.
Contains FBS, DMSO, glucose, salts
Buffering capacity pH = 7.2 to 7.6
Applications & Validation
The cells preserved in our CM-1 freeze medium can be used for cell counting, viability and cryopreservation, cell culture, mammalian cell culture, gene expression analysis and genotyping, in vitro transcription, and polymerase chain reactions. Each batch's efficacy is evaluated using CHO-K1 cells. Each batch is tested for pH, osmolality, sterility, and endotoxins to ensure high quality.
- A Gentle Alternative to Trypsin
Accutase is a cell detachment solution that is revolutionizing the cell culture industry. It is a mix of proteolytic and collagenolytic enzymes that mimics the action of trypsin and collagenase. Unlike trypsin, Accutase does not contain any mammalian or bacterial components and is much gentler on cells, making it an ideal solution for the routine detachment of cells from standard tissue culture plasticware and adhesion coated plasticware. In this blog post, we will explore the benefits and uses of Accutase and how it is changing the game in cell culture.
Advantages of Accutase
Accutase has several advantages over traditional trypsin solutions. Firstly, it can be used whenever gentle and efficient detachment of any adherent cell line is needed, making it a direct replacement for trypsin. Secondly, Accutase works extremely well on embryonic and neuronal stem cells, and it has been shown to maintain the viability of these cells after passaging. Thirdly, Accutase preserves most epitopes for subsequent flow cytometry analysis, making it ideal for cell surface marker analysis.
Additionally, Accutase does not need to be neutralized when passaging adherent cells. The addition of more media after the cells are split dilutes Accutase so it is no longer able to detach cells. This eliminates the need for an inactivation step and saves time for cell culture technicians. Finally, Accutase does not need to be aliquoted, and a bottle is stable in the refrigerator for 2 months.
Applications of Accutase
Accutase is a direct replacement for trypsin solution and can be used for the passaging of cell lines. Additionally, Accutase performs well when detaching cells for the analysis of many cell surface markers using flow cytometry and for cell sorting. Other downstream applications of Accutase treatment include analysis of cell surface markers, virus growth assay, cell proliferation, tumor cell migration assays, routine cell passage, production scale-up (bioreactor), and flow cytometry.
Composition of Accutase
Accutase contains no mammalian or bacterial components and is a natural enzyme mixture with proteolytic and collagenolytic enzyme activity. It is formulated at a much lower concentration than trypsin and collagenase, making it less toxic and gentler, but just as effective.
Efficiency of Accutase
Accutase has been shown to be efficient in detaching primary and stem cells and maintaining high cell viability compared to animal origin enzymes such as trypsin. 100% of cells are recovered after 10 minutes, and there is no harm in leaving cells in Accutase for up to 45 minutes, thanks to autodigestion of Accutase.
In summary
In conclusion, Accutase is a powerful solution that is changing the game in cell culture. With its gentle nature, efficiency, and versatility, Accutase is the ideal alternative to trypsin. If you are looking for a reliable and efficient solution for cell detachment, Accutase is the solution for you.